Product(s)
penergetic p
penergetic b
Partner
Ricardo Bemfica Steffen and Gerusa Pauli Kist Steffen PhD in Soil Science BRAZIL
Customer
Scientific Publication: In Vitro Activation of Microbial Growth in Low Frequency Electromagnetic Fields
"Significant increases in fungal and bacterial growth were observed when using the commercial biostimulator in culture medium."
Publication of Ricardo Bemfica Steffen, Gerusa Pauli Kist Steffen & Joseila Maldaner in the Journal of Agriculture and Environmental Sciences, June 2020, Vol. 9, No. 1, pp. 1-7
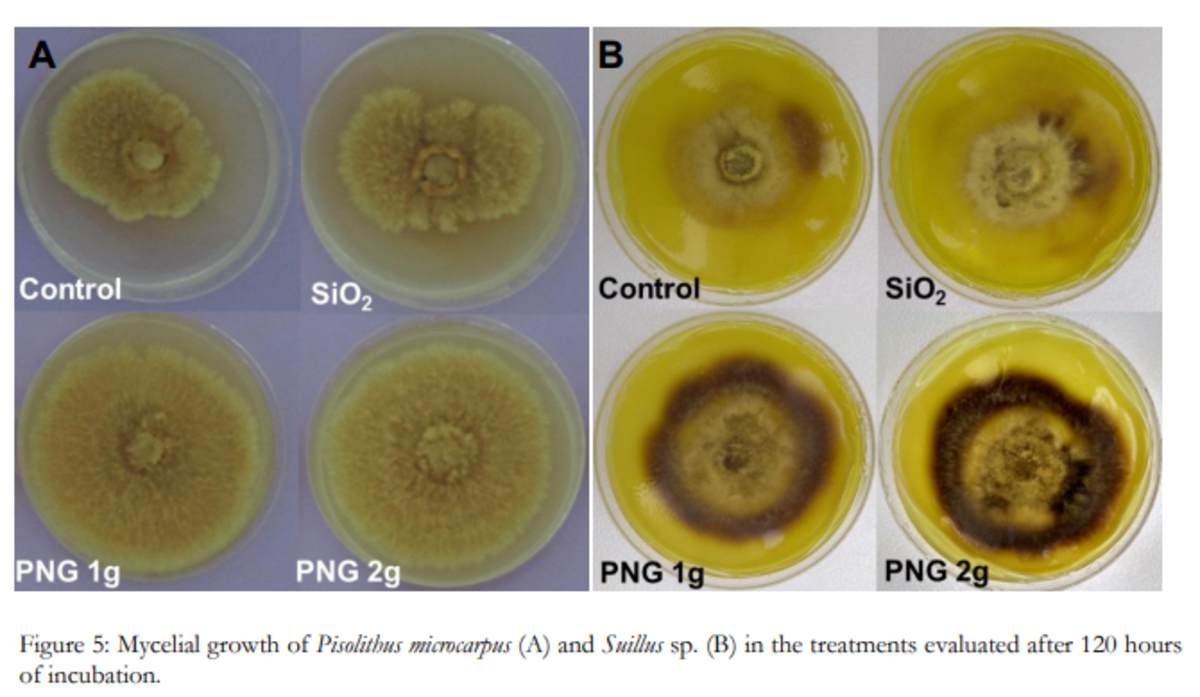
Mycelial growth of Pisolithus microcarpus (A) and Suillus sp. (B) in the treatments evaluated after 120 hours of incubation with penergetic (PNG1 and PNG 2) and without penergetic (Control and SiO2)
Abstract
The advances in productivity and product quality observed in world agriculture lead to the use of new technologies capable of meeting current demands. The use of low frequency electromagnetic fields has shown significant results. In this context, the objective of this study was determine the effects of Penergetic technology on the direct stimulation of beneficial soil microorganisms under in vitro conditions. Mycelial growth and conidial formation of Trichoderma asperelloides, mycelial growth of ectomycorrhizal fungi Pisolithus microcarpus and Suillus sp. and the multiplication of bacterial cells of Bradyrhizobium japonicum species in culture medium with and without the addition of Penergetic. Significant increases in fungal and bacterial growth were observed when using the commercial biostimulator in culture medium. The use of sustainable technological tools represents a viable path because it consists of a solid and efficient management alternative capable of increasing the stimulus to biological development in the soil and, consequently, to agricultural productivity in an environmentally and sustainable safety.
Study authors: Ricardo Bemfica Steffen, Gerusa Pauli Kist Steffen & Joseila Maldaner
Method
The direct effect of the technology was evaluated on two groups of fungi and one group of bacteria, all naturally occurring in the soil and widely distributed worldwide. The fungus Trichoderma asperelloides (biological control agent and plant growth promoter), two species of ectomycorrhizal fungi Pisolithus microcarpus and Suillus sp. (symbiotic fungi of forest species) and the bacterium Bradyrhizobium japonicum (biological nitrogen fixing bacteria) were used for this study.
Four treatments were evaluated:
- Control control treatment (using unchanged culture media)
- SiO2 silicon dioxide treatment (addition of commercial SiO2 in the culture medium)
- PNG 1g and PNG 2g two doses of Penergetic p plant (1 and 2 grams of Penergetic per liter of medium).
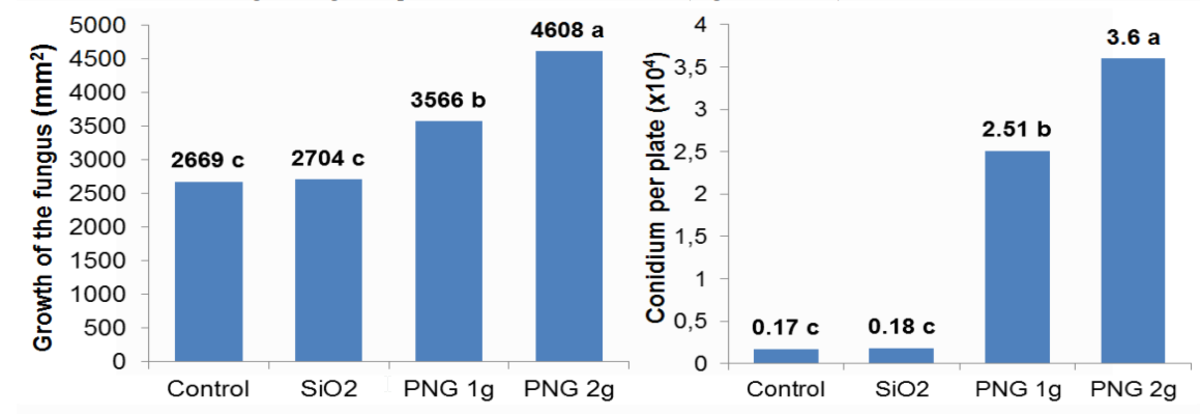
Figure 1: Trichoderma asperelloides growth determined by sporulation border diameter and number of conidium per plate. *Means followed by the same letter do not differ by the Tukey test at 5% probability.
Results and discussion
The results obtained in controlled environment microbial stimulus assays demonstrated the incremental effect on T. asperelloides (biocontrol agent and plant growth promoter), P. microcarpus and Suillus sp. (ectomycorrhizal fungi) and the bacterium B. japonicum. These results demonstrate that the use of penergetic p plant product has direct benefit on the growth and biological activity of the evaluated microorganisms.
Silicon dioxide, a substrate used by Penergetic technology, did not result in a significant increase in fungal development without the industrial process. The authors observed SiO2 stimulation potential on biological activity. However, when electromagnetic bioprogrammed SiO2 (Penergetic Plant) was used, sporulation was anticipated and the number of conidia per plate increased.
More details in the study to download below.
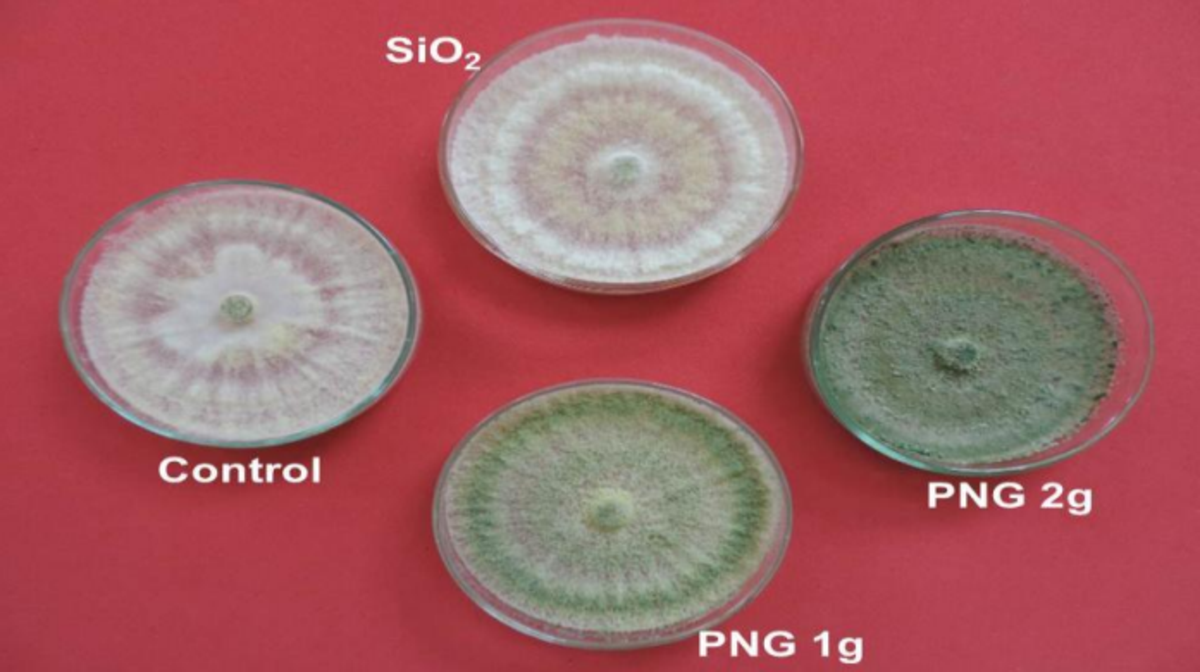
Figure 2
Mycelial growth and sporulation intensity
Figure 2: Mycelial growth and sporulation intensity of Trichoderma asperelloides in the treatments evaluated after 120 hours of incubation.
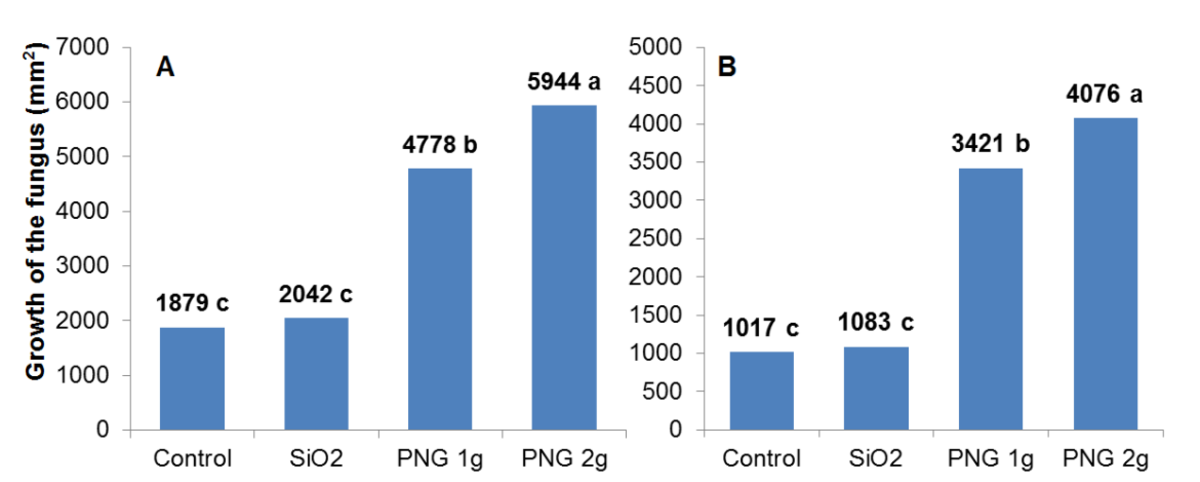
Figure 3
Fungal growth
Figure 3: Fungal growth of Pisolithus microcarpus (A) and Suillus sp. (B) determined by border diameter.
*Means followed by the same letter do not differ by the Tukey test at 5% probability.
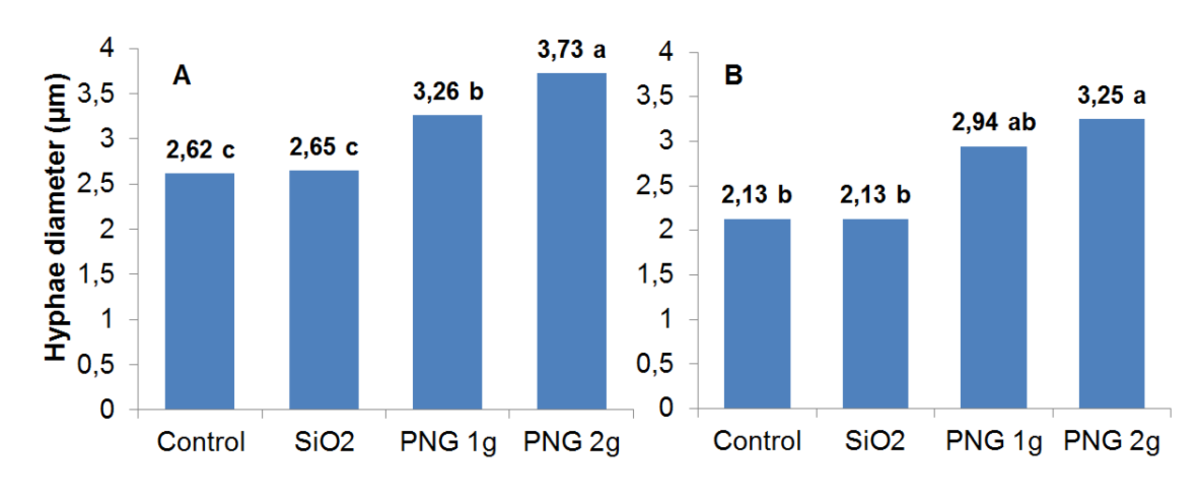
Figure 4
Average hyphae diameter
Figure 4: Average hyphae diameter (µm) of ectomycorrhizal fungi (A) Pisolithus microcarpus and (B) Suillus sp. in the different treatments. *Means followed by the same letter do not differ by the Tukey test at 5% probability.
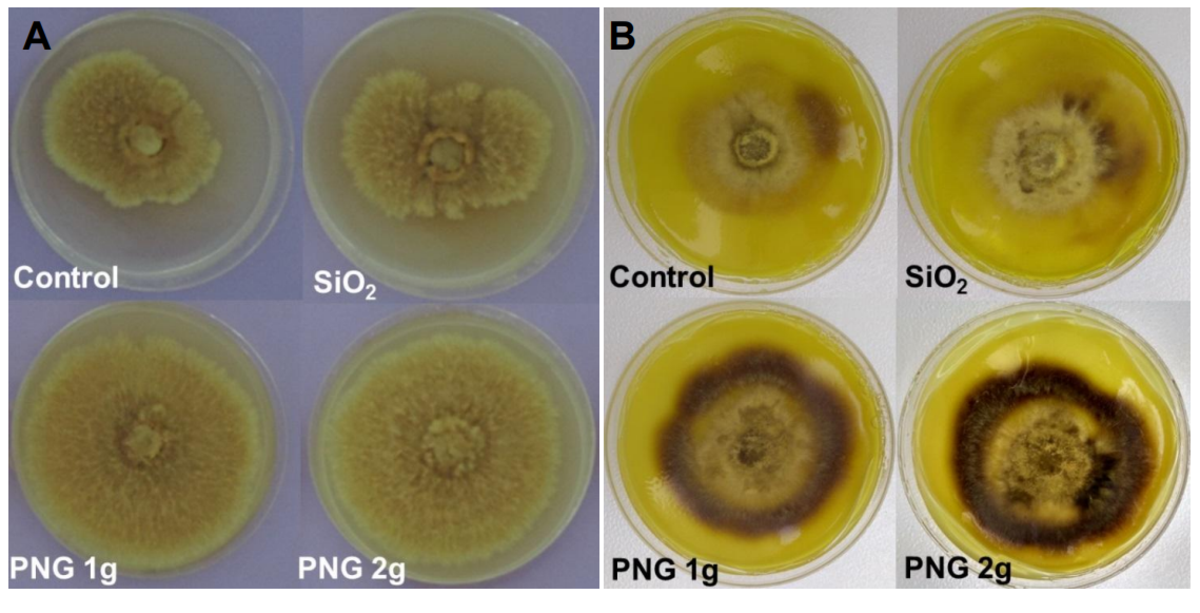
Figure 5
Mycelial growth
Figure 5: Mycelial growth of Pisolithus microcarpus (A) and Suillus sp. (B) in the treatments evaluated after 120 hours of incubation.
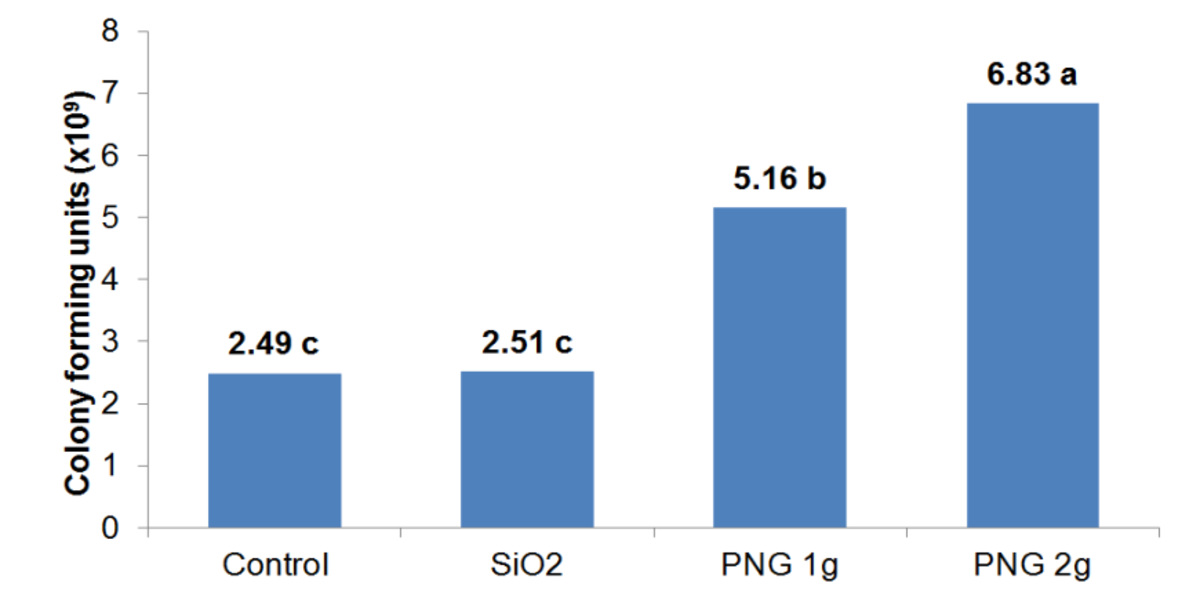
Figure 6
Colony Forming Units (CFU)
Figure 6: Colony Forming Units (CFU) of the Bradyrhizobium japonicum per milliliter of suspension.
*Means followed by the same letter do not differ by the Tukey test at 5% probability.